A Brief Colonial History Of Ceylon(SriLanka)
Sri Lanka: One Island Two Nations
A Brief Colonial History Of Ceylon(SriLanka)
Sri Lanka: One Island Two Nations
(Full Story)
Search This Blog
Back to 500BC.
==========================
Thiranjala Weerasinghe sj.- One Island Two Nations
?????????????????????????????????????????????????Thursday, October 27, 2016
Is Janet Woodcock, MD, Director of FDA Guilty of Medical Malpractice?
How many lives could have been saved if she had heeded AG Blumenthal's petition?
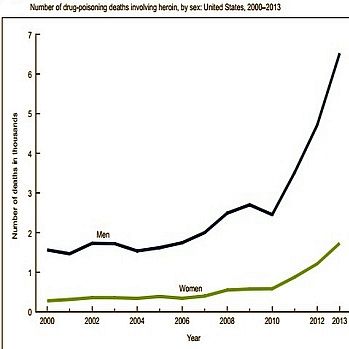
In 2000-2013 the rate for heroin-related overdose deaths was highest among adults aged 25-44.
|
(MYRTLE BEACH, S.C.) - Janet
Oct-24-2016
Woodcock, MD, is the director of the Center for Drug Evaluation and
Research (CDER) at the Food and Drug Administration (FDA). The center
makes sure that safe and effective drugs are available to improve the
health of people in the United States.
In view of the prescription opioid epidemic evolving into a Tsunami of
deaths and addictions, did Dr. Woodcock contribute to the volcanic
eruption of the epidemic by violating rules of the FDA?
On January 24, 2004 then Attorney General (AG) Richard Blumenthal of
Connecticut (now US Senator of Connecticut) submitted a Citizen Petition
(CP) to the FDA entitled "Petition to Require Purdue Pharma L.P. to
Revise the Labeling of OxyContin Tablets to Strengthen Warnings of the
Greater Potential for Developing Side Effects and Adverse Drug Reactions
Due to Prescribing Dosing Frequencies in Excess of the Recommended
Guidelines".
Although the FDA has two conflicting dates on their website as to the
time frame to respond to a Citizen Petition of 5 months or 6 months,
Janet Woodcock, MD did not respond to Blumenthal's 2004 CP until 2008.
Way beyond the 5 to 6 months the FDA is required to respond. Provided
below is a link to Woodcock's response to Blumenthal -- all 18 pages of
it.
One of the comments Dr. Woodcock stated in her reply was that
".....although higher total daily dosages were associated with fatal
outcomes, it cannot be concluded that the higher total daily dosages are
causally associated with fatalities given the other variables have not
been measured."
Further she writes "...there is no maximum dose for opioids."
Recently the Center for Disease Control (CDC) recommendations on
prescribing opioids for chronic pain stated as follows "Benefits of
high-dose opioids for chronic pain are not established."
The complete opposite of Woodcock's 2008 quotation of "no maximum dose".
So, answer me this Dr. Woodcock -- how many lives could have been saved when AG Blumenthal sent the FDA his Citizen Petition in 2004 if you had acted on it in a timely and scientific manner?
What do you think? Hundreds? Thousands? Tens of Thousands? Do you
believe that you are responsible for this out of control prescription
opioid epidemic by your inaction? Or worse, could you be guilty of
medical malpractice?
In Blumenthal's CP to the FDA, he references an interview he conducted
with a woman in Iowa. She agreed to have her name used in his petition.
In 1999, the woman's physician prescribed her OxyContin 20 mgs twice a
day. She complained to her doctor of many side effects and he treated
what she called "addiction" to OxyContin by increasing her dosage.
In May 2002 acting on the advice of her orthopedic surgeon, she
attempted "to wean herself from the drug." This attempt was short-lived
and in September 2002, she voluntarily admitted herself into a drug
treatment facility in Illinois where she spent two and one half days
before leaving the program.
Later that month she spent one week going "cold turkey" in the basement
of her parents' home before she was finally successful in coming off
OxyContin.
I know this story first hand Dr. Woodcock, because I put this woman who
was "addicted" to OxyContin in contact with AG Blumenthal and I knew her
story quite well. So what I would like to know from you, Dr. Woodcock,
is this:
How could you say in your September 9, 2008 reply to AG Blumenthal's CP
-- almost 5 years after the deadline indicated on the FDA website to
respond to a Citizen Petition -- that the woman referenced in the CP who
had been interviewed by Blumenthal "ultimately weaned herself from the
medication."
Really? Maybe you should look up the medical definition of "weaning".
Going cold turkey on a basement floor for a week is not weaning. We both
know that.
As a medical doctor, how could you state how this woman was treated or
ceased to take OxyContin? You never interviewed her or examined her.
Careless on your part Dr. Woodcock or a clear case of medical
malpractice?
Citizen Petition
________________________________________________ Date:____________ The undersigned submits this petition under __ (relevant statutory sections, if known) of the __ (Federal Food, Drug, and Cosmetic Act or the Public Health Service Act or any other statutory provision for which authority has been delegated to the Commissioner of Food and Drugs) to request the Commissioner of Food and Drugs to__ (issue, amend, or revoke a regulation or order or take or refrain from taking any other form of administrative action).
Off the FDA website -
Section 505(q)(1)(F) governs the timeframe for final Agency action on a
petition. Under this provision, FDA shall take final Agency action on a
petition not later than 150 days after the date on which the petition
is submitted. The 150-day period is not to be extended for any reason,
including any determination made under section 505(q)(1)(A) regarding
delay of approval of an application, the submission of comments or
supplemental information, or the consent of the petitioner.
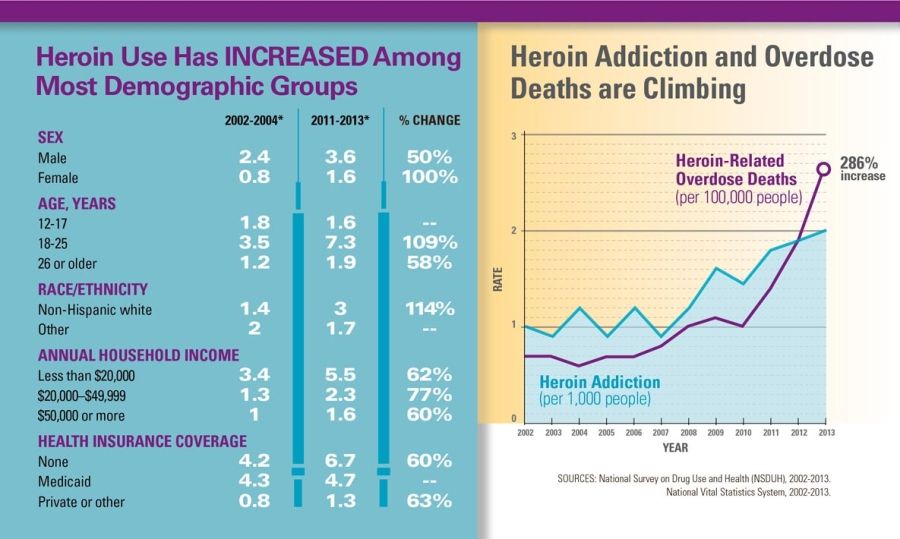
Next in a series: How Dr. Woodcock's statements in her 2008
reply to AG Blumenthal's Citizen Petition furthered the prescription
opioid epidemic leading to an unprecedented rise in heroin deaths.
SEE ALSO:

